by Dr Jay Lu
In our earlier post, we touched base on the applications and benefits of cardiac optical mapping, but the physiological significance still leaves room to be explored.
As we discussed, the optical mapping technique was developed to help us determine the electrical properties of multicellular cardiac preparations, ranging from iPSC-cardiomyocyte to intact heart ex vivo and in vivo. The secret sauce of optical mapping is to grasp high-speed fluorescence-based imaging with various probes for transmembrane potential (Vm), intracellular calcium transient, and other critical physiological parameters.
What is the physiological significance of optical mapping?
Before we dive into the answer, it is important to understand that the concept of excitation-contraction coupling is characterized by action potential and calcium transient. It represents the process by which an electrical excitation (action potential) leads to contraction of cardiac muscle cells (1). Calcium plays a key role in coupling electrical excitation to physical contraction by cycling in and out of the myocyte’s cytosol during each action potential (1).
Thus, it is imperative to map both membrane potential and calcium transient simultaneously from the same location on a Langendorff perfused heart. This simultaneous mapping can help us better understand the mechanisms underlying arrhythmias, metabolic diseases, the pathology of heart failure, and altered ion channel and gap junction expression (9).
Mapping of cardiac arrhythmias
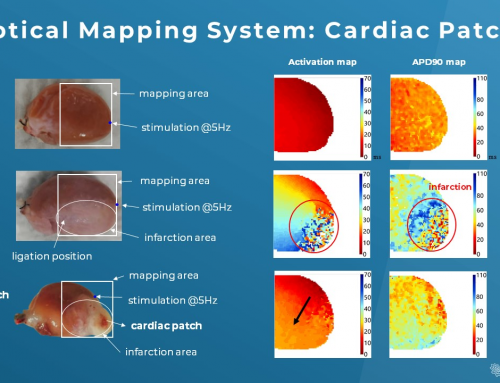
Arrhythmia is one of the main areas for optical mapping which can help decipher complex patterns of cardiac excitation propagation (2). In fact, the advantages of high spatiotemporal resolution, wide field mapping, and high sensitivity to transmembrane potential in optical mapping enable detailed and precise mapping of action potential initiation and propagation during disease state, i.e. activation pattern and repolarization pattern (3).
Here are some well-known electrophysiological parameters of optical mapping:
- Action potential & calcium transient
- Action potential duration
- Visualization of activation (isochronal map)
- Conduction velocity
- Dispersion of conduction
To understand what happens in the abnormal wave initiation or propagation across the heart is essential for creating anti-arrhythmic therapeutic strategies and reducing cardiovascular mortality. Clinically, “reentry” is the basis of most arrhythmias encountered due to the action potential propagating in a circus-like closed loop manner. Common reentry conditions include atrial and ventricular fibrillation and ventricular tachycardia following myocardial ischemia or infarction (4). In fact, further dynamic optical mapping data has surfaced in arrhythmia research and made invaluable contributions to our understanding of mechanisms of cardiac arrhythmias (2,4,5,6).
Calcium plays a major role in arrhythmias
Normally, depolarization triggers calcium transient with a tightly regulated mechanism involving sarcoplasmic reticulum (SR) and calcium-induced calcium release (CICR) processes (10). However, defective calcium handling may activate calcium-dependent currents that affect the action potential duration and trigger a spontaneous membrane depolarization (7,8).
Thus, calcium handling abnormalities may contribute to arrhythmogenesis (the development of arrhythmia) through, for instance, ischemia, reperfusion-arrhythmias, generating early and delayed afterdepolarizations, and torsades de points seen in patients with the long QT syndrome (11). Furthermore, it has been well demonstrated that calcium overdrive may spawn electromechanical alternans and an increase in action potential duration restitution curves steepness (12). These two phenomena are closely associated with arrhythmogenesis.
Final words
This post provides a brief overview of the physiological basis and clinical significance of cardiac optical mapping. Optical mapping techniques have changed the investigation methods of cardiac physiology and have been at the forefront of scientific discoveries, especially with the ability to monitor physiological parameters in intact hearts.
One cannot stress enough the importance of simultaneous measurements of action potential and calcium transient from several hundreds of sites in the heart. This will empower researchers to map all phases of electrical activity, including activation and repolarization, and gain new insights into the mechanisms of arrhythmias with more physiological sophistication and clinical relevance.
Reference:
- Bers DM. Cardiac excitation-contraction coupling. Nature. 2002 Jan 10;415(6868):198-205.
- Efimov IR, Nikolski VP, Salama G. Optical imaging of the heart. Circ Res. 2004 Jul 9;95(1):21-33
- Berenfeld O, Efimov I. Optical Mapping. Card Electrophysiol Clin. 2019 Sep;11(3):495-510.
- Davidenko JM, Pertsov AV, Salomonsz R, Baxter W, Jalife J. Stationary and drifting spiral waves of excitation in isolated cardiac muscle. Nature. 1992 Jan 23;355(6358):349-51.
- Davidenko JM, Kent PF, Chialvo DR, Michaels DC, Jalife J. Sustained vortex-like waves in normal isolated ventricular muscle. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8785-9.
- Gray RA, Pertsov AM, Jalife J. Spatial and temporal organization during cardiac fibrillation. Nature. 1998 Mar 5;392(6671):75-8. doi: 10.1038/32164. Erratum in: Nature 1998 May 14;393(6681):191.
- Laflamme MA, Becker PL. Ca2+-induced current oscillations in rabbit ventricular myocytes. Circ Res. 1996 Apr;78(4):707-16..
- Lakatta EG, Guarnieri T. Spontaneous myocardial calcium oscillations: are they linked to ventricular fibrillation? J Cardiovasc Electrophysiol. 1993 Aug;4(4):473-89.
- London B. Cardiac arrhythmias: from (transgenic) mice to men. J Cardiovasc Electrophysiol. 2001 Sep;12(9):1089-91.
- Fabiato A, Fabiato F. Use of chlorotetracycline fluorescence to demonstrate Ca2+-induced release of Ca2+ from the sarcoplasmic reticulum of skinned cardiac cells. Nature. 1979 Sep 13;281(5727):146-8.
- Landstrom AP, Dobrev D, Wehrens XHT. Calcium Signaling and Cardiac Arrhythmias. Circ Res. 2017 Jun 9;120(12):1969-1993.
- Kanaporis G, Blatter LA. The mechanisms of calcium cycling and action potential dynamics in cardiac alternans. Circ Res. 2015 Feb 27;116(5):846-56. doi: 10.1161/CIRCRESAHA.116.305404.