by Dr Jay Lu
Hydroxychloroquine (HCQ) has been touted as a promising and effective treatment for patients with the COVID-19 infection. The drug is often recommended to be used in conjunction with azithromycin, an antibiotic. In this article, we will have a closer look at recent studies on hydroxychloroquine, which may shed light on why the W.H.O. has halted clinical trials of HCQ for COVID-19.
While there is some reported beneficial effect with hydroxychloroquine (HCQ) and azithromycin (AZM) for SARS-CoV-2, more and more studies suggest no difference in viral clearance and perhaps a cause in adverse effects with this treatment in high doses. This does not address those advocates of HCQ studies which were not peer reviewed and greatly challenged by the scientific community.
More Solid Evidence…
In particular, a multinational registry analysis in the Lancet, a peer-reviewed medical journal, showed that people taking the drug were at higher risk of death and ventricular arrhythmia than those who were not (1). This study did not observe a beneficial effect of HCQ when used alone or with AZM for COVID-19.
Moreover, a clinical cohort study also supported the idea that HCQ can alter the electrical activation of the heart in a way that increases its susceptibility to life-threatening rhythm disturbances. In one report, the QTc interval increased above the critical level of 500 milliseconds in 20% of treated patients. Lengthening of the QTc interval was four times greater in patients who had received azithromycin in addition to HCQ. One patient treated with the drug combination developed torsade de pointes (2). The QT prolongation induced by HCQ in patients with COVID-19 has also been confirmed by the latest systematic review (3).
The Mechanism Behind It…
Interestingly, a recent electrophysiological study (4) supports the idea of above clinical observations and uncovers the mechanism behind it. Wang et. al. looked at all the relevant ion channels and found that HCQ can block a variety of cardiac ion channels in animal models, such as hERG, Kir2.1 and hNav1.5. Notably, hERG is a critical component of the Comprehensive in Vitro Proarrhythmia Assay (CiPA) for drug safety assessment.
Subsequently, the study further explored how the blockades are translated into abnormal or sometimes lethal cardiac complications, including a slowed heart rate, prolongation of action potential duration (APD) and intracellular Ca2+ transient, and abnormal ventricular conduction.
The adverse effects of HCQ are amplified with the addition of AZM, which elicited electrical alternans, ventricular tachycardic episodes, and reentrant excitation. In the worst scenario, these cardiac abnormalities could result in Torsade de Pointes (ventricular tachycardia), which can then develop into sudden cardiac death.
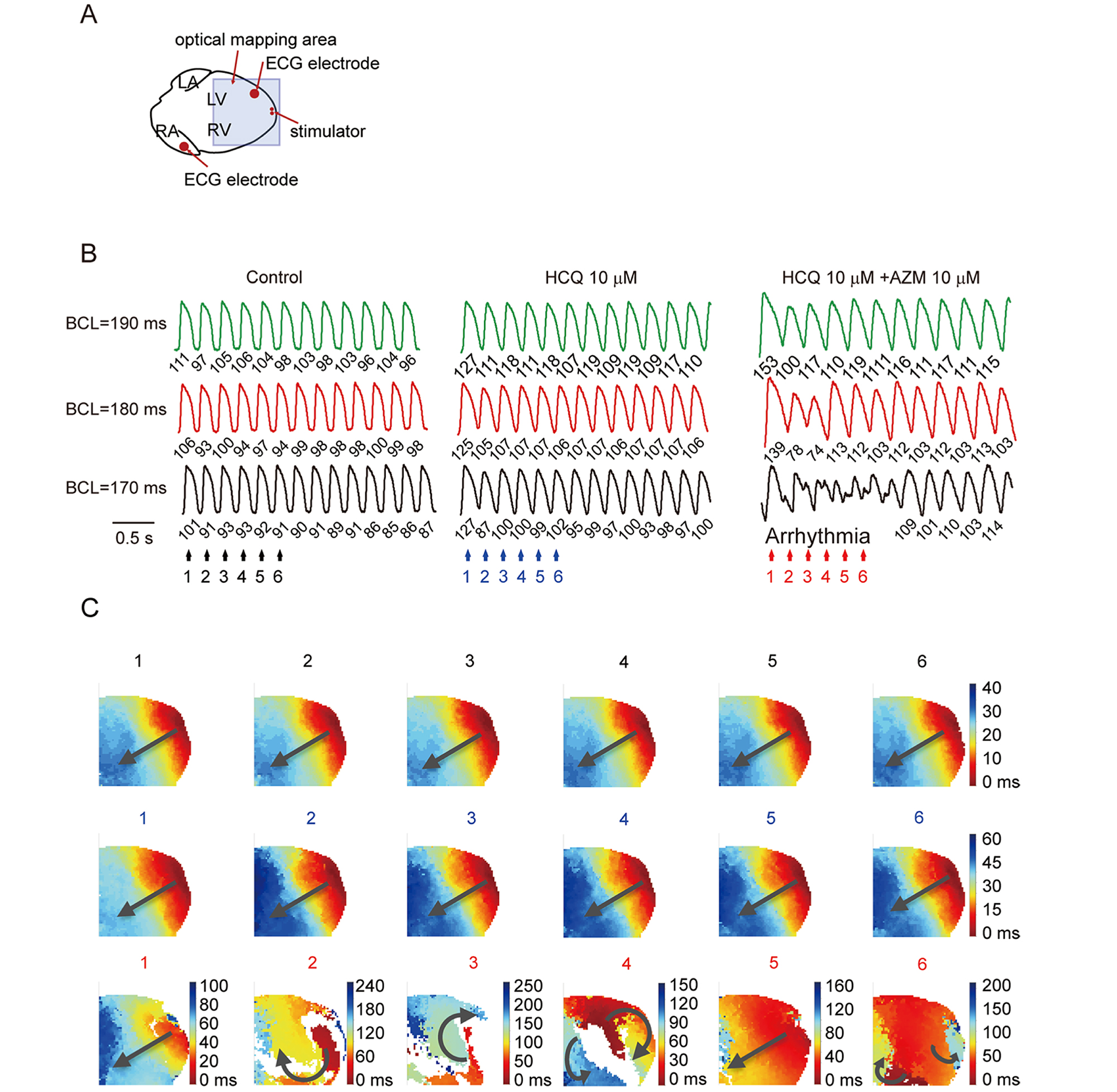
Figure. Voltage RH237 optical mapping of re-entry and ventricular arrhythmic tendency in isolated perfused hearts. (A) Recording and programmed stimulation configuration of optical mapping systems by MappingLab. (B) Optical action potential traces obtained under programmed pacing at 190 ms, 180 ms and 170 ms cycle lengths (CLs), before (control) and following addition of 10 μM HCM, and 10 μM HCM with added 10 μM AZM. (C) Corresponding voltage maps obtained at the numbered color-coded timepoints (n hearts). Source: https://www.biorxiv.org/content/10.1101/2020.05.21.108605v3
The Bottom Line
Through a deeper understanding of the mechanism of HCQ with AZM in a physiological condition, it would be extremely helpful for scientists or policy makers to decide whether the drug should be prescribed for a disease. Drug toxicity screening is an important stage that acts as a gatekeeper for all of us.
Reference
- https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)31180-6/fulltext
- https://jamanetwork.com/journals/jamacardiology/fullarticle/2765631
- https://www.heartrhythmjournal.com/article/S1547-5271(20)30431-8/fulltext
- https://www.biorxiv.org/content/10.1101/2020.05.21.108605v3